The Story of pH
The Story of pH Made Easy
There are numerous publications for professionals that cover the theory and studies on the difficult subject of pH. However, there are very few, if any, books on conductivity written for the layman.
“The Story of pH Made Easy” explains in plain language what pH is and how pH affects our lives. We hope you will have a clearer understanding of pH after reading this story.
Preface
You have probably at least heard of or seen the term pH, It has become something of a household word. In advertising, there has been mention of things like “the pH of your skin” and “pH of your diet.” You may also remember experiments in school using litmus paper to see if a solution was acidic or alkaline.
Finding the value for pH is essential in determining an important characteristic of a solution.
So, pH is just a unit to represent one characteristic of a solution, just as the meter is a unit of length.
So, what kind of characteristic is represented by pH?
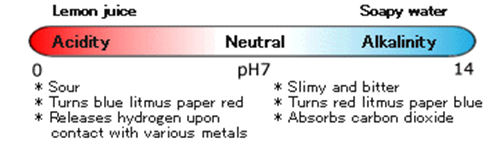
Acidity or Alkalinity
Lemon juice tastes sour. Soapy water is slimy and tastes somewhat bitter. If you dip blue litmus paper into lemon juice, it turns red. If you dip it into soapy water, it turns blue. Because lemon juice is acidic, it makes blue litmus paper turn red.
Because soapy water is alkaline, it turns red litmus paper blue.
And pH represents the acidity or alkalinity of a solution, such as your lemon juice or soapy water.
In the chart below, you can see that pure, neutral water has a pH of 7. Any solution with a ph below 7 is acidic, and if the ph is above 7, then it’s alkaline.
Facts About pH Values
Did you know that blue ink had such acidity? It will help you if you know various pH values in your life !

Anecdotes
Stomach Digesting Stomach?
Barbecue and beer is such a nice combination, but if a stomach can digest meat, what keeps it from digesting itself? The human stomach uses hydrochloric acid and enzymes such as pepsin to break down and digest proteins that have entered the stomach. Gastric mucus, besides providing lubrication, lines the stomach wall and protects it from being digested by the stomach’s own gastric juices. The pH in our stomachs is usually around 2. If this pH is too low (strongly acidic), the stomach wall does become vulnerable. The health of our stomachs depends on pH.
Horses Run on Grass
There is a saying in England, home of thoroughbreds, “Horses run on grass,” meaning high-quality grass is required in raising good thoroughbreds.
The soil in Japan has acidity of pH 4.2-5.5, with a much lower content of calcium, phosphates and magnesium than that in England, where soil pH runs up to 6.5 or 7. So, wild grass in Japan is not conducive to raising horses with strong bones. Efforts have been continuously made to weaken the acidity of the soil by adding magnesium oxide lime and calcium carbonate.
A thoroughbred born and raised in Japan may win a derby championship in England yet.
To Your Health! Viva, Alkaline!!
People are becoming more health-conscious. Healthy foods are very popular these days. By the way, many people misunderstand “acid food, alkaline food.” This word does not refer to the pH of the food itself but to the pH of the food when absorbed by the body. For example, let’s talk about lemons. The lemon itself is quite acidic. However, it is called an alkaline food. Human bodies are most healthy when mildly alkaline.
Therefore, we should eat not only acid foods such as meat and fish but also alkaline foods: FRUITS AND VEGETABLES !
Is the Rain in Our Cities Acidic?
Have you ever experienced eye discomfort or holes in your pantyhose when it rained? You have acid rain to thank. Or, more accurately, its cause: SO2 and NOx belched out by factories and motor vehicles combine with water to form the sulfuric and nitric acid in acid rain. The pH of rain falling on the countryside with clean air will be around 6–mild acidity.
However, in big cities, the pH of rain, especially when it first starts to fall, can run as high as 4.
Are Hydrangeas Nature’s Litmus Paper?
As you know, the hydrangea is a flower of striking color variety–white, blue or pink. Did you know this color variation was related to the pH of the soil? It is said that acid soil with a pH of 4.5 to 5 yields bright blue hydrangeas, but the color becomes close to red as the acidity becomes weak. Color wise, this is just the opposite of litmus paper. You might call hydrangeas nature’s litmus paper. And they’re a lot nicer to look at than store-bought litmus paper!
Measurement of pH in Many Fields
Textiles, Dyeing, Paper, Pulp, Chemistry, Oil Refining, Metals, Minerals, Electricity & Electrochemistry
Measuring pH is essential not only in finding the chemical characteristics of a substance but also as the first step toward managing chemical reactions. Currently, pH measurement is used in various fields, including nearly all industries that deal with water, not only the chemical industry, but public organizations, agriculture and fishery-related industries and biological industries, as well.
We would like to discuss fields where pH measurement is important and why.
Textiles, Dyeing
In the textile industry, measuring pH is important in product testing, such as how a fabric reacts to things like perspiration, especially when developing synthetics, to ensure safety and durability.
Of course, pH measurement is indispensable in the process of dyeing, because permanence and processing speed depend on the pH of the dye bath, which is automatically adjusted to maintain proper pH,
Also, pH measurement is applied when creating artificial perspiration to be used in color-change testing.
Paper, Pulp
Measurement of pH is carried out in the manufacturing processes of paper and pulp more often and continuously than any other industry. To reduce the consumption of chemicals and prevent corrosion of equipment, pH is controlled in the process of digestion, bleaching, creating of pulp to the manufacture of finished paper material products. Also, the durability and drying speed depends on the pH of the paper itself. Therefore, pH is strictly checked and adjusted as a vital part of quality control.
Chemistry
 As pH measurement is essential for control of chemical reactions, it is carried out in the course of nearly all production of chemical products, be it plastics, fertilizers, electronic industrial materials such as semiconductors, cements, or glass. In order to optimize the desired reaction and to prevent unwanted reactions, controlling the pH of solutions is very important.
As pH measurement is essential for control of chemical reactions, it is carried out in the course of nearly all production of chemical products, be it plastics, fertilizers, electronic industrial materials such as semiconductors, cements, or glass. In order to optimize the desired reaction and to prevent unwanted reactions, controlling the pH of solutions is very important.
For example, in the manufacturing process for plastics, pH is strictly managed in the processes of producing long-chain molecular products, especially the processes of polymerization and condensation. Also, pH is controlled for efficient production of chemical fertilizers such as nitrogen fertilizers, potash fertilizers and phosphate fertilizers.
Also, pH measurement is very important both in the mixing process of silicate in the case of cement and in combining processes at high temperature in the case of glass manufacturing. The transparency of finished glass is also influenced by pH.
Oil Refining
In oil refining, measurement of pH is carried out in the desulfurization process.
Metals and Minerals
Each metal tends to dissolve in solution of a certain pH by nature. Based on this characteristic, when extracting a particular material from crude ore or mixed metal, pH is controlled so as to extract only the desired metal without dissolving the slag. For example, if you place a mixture of copper and zinc in acid electrolytic solution and electrolyze it, only copper is separated out at the negative electrode.
Electricity, Electrochemistry
In this field, pH measurement is applied to plating, etching of metal surfaces and the manufacture of batteries.
Control of the pH of a plating solution greatly affects the finish. Without proper control of the pH of a plating solution, the finished plating will be likely to peel and will not have the optimum color and luster. Anodic oxidation processing is used in coating cooking utensils to produce a coating like the film that forms on aluminum objects, and controlling the pH of the processing solution is critical to achieving the desired finish. In advanced factory painting processes, pH is a vital factor in achieving paint finishes of the quality required for manufactured goods.
Electric Power, Gas, Medicines, Cosmetics, Fisheries, Food, Brewing, Agriculture & Animal Husbandry
Electric Power, Gas
The pH of the water used in all the boilers, including those large boilers used at thermal power plants is maintained above 8 in order to prevent corrosion of pipes. The boiler water is also kept alkaline to prevent corrosion. However, if it becomes too alkaline, a calcium oxide is formed, which is caustic and adheres to the wall of the boiler. Thus, pH is maintained at about 9.4 in the boilers of thermal power plants.
With today’s high-performance, high-efficiency boilers, control of the water becomes critical, and pH control of boiler water is conducted not only at power plants, but in a wide variety of fields.
In the gas industry, the pH of the water in liquefied gas is controlled in the mixing process to enhance caloric potential and prevent corrosion of tanks and pipes.
Medicines and Cosmetics
In the pharmaceutical and cosmetics industries, pH is measured in order to check chemical reactions in production. Speed of reactions depends on the pH of a solution, and the end point of a reaction can be estimated by knowing the pH.
For example, in the production of antibiotics, pH must be controlled in the fermentation processes to maintain high yield and antibacterial properties.
Also, as medicines and cosmetics are taken into the human body or applied to human skin, the strictest quality control is required.
If there is big difference in pH between skin and cosmetic products, the results could be disastrous. If a medicine is of the wrong pH, that medicine could be a poison.
Fisheries
The pH balance of water can greatly influence the marine life, and is a major factor when measuring water quality of fishing grounds and fish farms for seaweed, cultured pearls, yellowtail and eel. In fact, pH is the first factor examined when selecting a location for fish farming, and is often the first sign of an abnormality in the fishing grounds and hatcheries. At the central fish market, the pH of the fish delivered from various fishing grounds is measured to check the freshness of the fish.
Food and Brewing
The food and beverage products that most require pH measurement are those whose production involves enzyme reactions, such as bread, liquor, beer, soy sauce, miso, cheese and milk products. This is because the action of enzymes is affected by pH, and each has an optimum pH critical to the results.
With some foods, pH is an important factor in taste and safety.
Each food has an optimum pH value, and if the pH value is too high or too low, it is likely that it won’t taste right or that safety has been compromised by some foreign substance. Every maker of processed food checks pH as part of the effort to make good-tasting food of consistent quality.
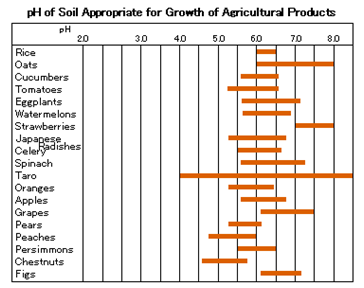
Agriculture, Animal Husbandry
Growth and development of plants is closely associated with pH. Each plant has a specific pH appropriate for its growth.
In the fields, measuring the pH of soil and water is important for increased production and control of growth of agricultural products.
Also, knowing the pH of culture solution is essential for tank farming, which is now popular. In the livestock industry, researchers measure the pH of animals’ blood and their feed and study its relationship with animal health and growth. Moreover, they check the pH of sperm when studying artificial insemination and when testing the freshness of meat and eggs.
Water Supply
Water Supply/Sewage
At filtration plants, water from rivers and lakes is disinfected with chlorine, and impurities are settled out and filtered out and removed with the aid of an agglutinating agent. At this stage, the pH of the water is maintained at a value appropriate for the action of the chlorine and the agglutinating agent.
Naturally, before this water is supplied to homes and commercial and public facilities, alkaline are added to neutralize it so it will be appropriate as drinking water, etc. At sewage-treatment plants, pH is measured not only in each process and at the time of the release after treatment, but also in adjustment of pH to optimize the activity of bacteria in sludge when using the activated-sludge method and treatment of generation of bubbles from the active agent.
Medical Organizations
Medical organizations measure pH to adjust reagents. They also measure the pH of such body fluids as blood, gastric juices and urine for medical research, medical testing and treatment.
Also, researchers at dental colleges measure pH when studying tooth decay. They have found that pH in the mouth begins to decrease five minutes after eating and that the fluid in the mouth begins to dissolve tooth enamel when pH reaches less than 2.5.
Example of Actual Measurement with an Electrode Inserted into the Stomach and Duodenum
The Living Body and pH
Gastric Juices (1.5-2.0)
Skin (4.5-6.0)
Duodenal Juices (5.0)
Urine (4.8-8.0)
Saliva (5.0-7.5)
Purulence Fluid (6.0-6.8)
Perspiration (7.0-8.0)
Tears (7.2)
Cerebral Fluid (7.3-7.5)
Breast Milk (6.6-6.9)
Blood (7.42)
Intestinal Juices (7.7)
Uterine Fluid (8.0-8.8)
Stomach Sweetbreads (8.0)
Environmental Pollution
With the public outrage and tough laws against water pollution caused by industrial waste water, all factories are now strictly controlling discharge of waste.
Currently, each local government designates water-quality standards and enforces restrictions. The pH of discharged water is one of the most measured items under water-quality regulations. Although pH does not necessarily indicate a particular kind of pollution, it is closely related with the survival of aquatic life. Abnormal pH can cause settling of halomorphic compounds and pollution of water.
Also, each factory is required to treat its waste water to conform to the quality standard for waste water. Measuring pH is important in this process, too. For example, at a plating plant, treatment efficiency is greatly affected by pH of the processing solution when removing cyanide or chromium from waste water.
Printing
Controlling the immersion liquid in offset printing, the most common printing method, involves very complex factors.
Adjustment of pH is one of the essential items, because it greatly affects the dryness of ink and color tone and color definition of photographic printing.
Miscellaneous
*Scientific criminal investigation by police
Science plays an important role in modern criminal investigation. Measuring pH is helpful in determining a victim’s time of death.
*Merchandise testing at department stores, consumer centers
Testing facilities of department stores and consumer centers conduct quality checks of merchandise so that consumers can buy, use and eat the merchandise without worry. Measuring pH can tell them the permanence of dye in a fabric or the freshness of food.
*Plant physiology
Measuring pH is essential for plant physiology, too. For example, pH is measured when experimenting with plant breeding and in the study of color change of flowers.
*Beauty Salons
For fashionable hair styles, cold-waving liquid plays an important role in achieving such beautiful waves. At beauty salons, pH is regulated in the cold-waving liquid according to hair type and wave.
*Golf Courses
Bright green lawns grow with grass of uniform height, and contribute to enjoyable golfing. To keep the lawns in top condition, greens keepers check the pH of the soil and provide necessary care accordingly.
*Photo Developing Labs
Controlling the pH of the developer is a very important part of faithful color reproduction–of skin tones, the bright red of a flower, and the blue of the sky. For color negatives, the pH value of developer varies from 3 to 12, depending on the developing process. A slight variance in pH can cause a difference in colors.
*Pharmacies
Pharmaceutical products seem indispensable in modern life.
Pharmacies not only mix drugs according to prescriptions, they must maintain quality. A pH meter is used in that effort.
*Building Cleaning
A clear blue sky seen through clean windows and shiny office floors will enhance the efficiency of your work. However, the precious wax will become cloudy if one does not completely remove the alkaline ingredient within the remover and detergent.
As you can see from this example, pH is an important factor in our daily lives.
*Swimming Pools
The concentration of residual chlorine and the pH value are important items in controlling the water in swimming pools.
In fact, pH is measured in more fields than we have room here to list.
Ways of measuring pH
The methods for measuring pH fall roughly into the following three categories:
(A) Indicator methods
(B) Metal-electrode methods (including the hydrogen-electrode method, quinhydron-electrode method and antimony-electrode method)
(C) Glass-electrode methods
A brief description of each method follows:
(1) Measuring pH using an Indicator
This category basically includes two methods: One involves comparing the standard color corresponding to a known pH with the color of an indicator immersed in the test liquid using buffer solution. The other method involves preparing pH test paper which is soaked in the indicator, then immersing the paper in the test liquid and comparing its color with the standard color. This method is simple, but prone to error. A high degree of accuracy cannot be expected.
* Various errors include;
– Error due to high salt concentration in the test liquid
– Error due to the temperature of the test liquid
– Error due to organic substances in the test liquid
(2) Hydrogen-Electrode Method
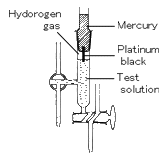
A hydrogen electrode is made by adding platinum black to platinum wire or a platinum plate. It is immersed in the test solution and an electric charge is applied to the solution and platinum black with hydrogen gas.
The hydrogen-electrode method is a standard among the various methods for measuring pH. The values derived using other methods become trustworthy only when they match those measured using hydrogen electrode method.
However, this method is not appropriate for daily use because of the effort and expense involved, with the inconvenience of handling hydrogen gas and great influence of highly oxidizing or reducing substances in the test solution.
(3) Quinhydron-Electrode Method
This method involves immersing the tip of a polished antimony rod into a test solution, also immersing a reference electrode, and measuring pH from the difference in potential between them. This method was once widely used because the apparatus is sturdy and easy to handle. However, its application is now quite limited because results vary depending on the degree of polish of the electrode, and reproducibility is low.
Note: Quinhydron solution of a certain pH is sometimes used to check whether an ORP meter is operating normally. The principle of the quinhydron electrode is applied in such a case.
(4) Antimony-Electrode Method
This method involves immersing the tip of a polished antimony rod into a test solution, also immersing a reference electrode, and measuring pH from the difference in potential between them. This method was once widely used because the apparatus is sturdy and easy to handle. However, its application is now quite limited because results vary depending on the degree of polish of the electrode, and reproducibility is low.
Note: This method is now used only in cases where a high degree of accuracy is not required (only for industrial use) and the test solution contains F-.
(5) Glass-Electrode Method
In this field, pH measurement is applied to plating, etching of metal surfaces and the manufacture of batteries.
This method is most widely used for pH measurement because the balancing time of electrical potential is short, it has high reproducibility, it is rarely affected by oxidizing and reducing agents, and it can measure pH of various solutions.
This method is used not only in industry but in a wide variety of fields.
Source by: Horiba
